send link to app
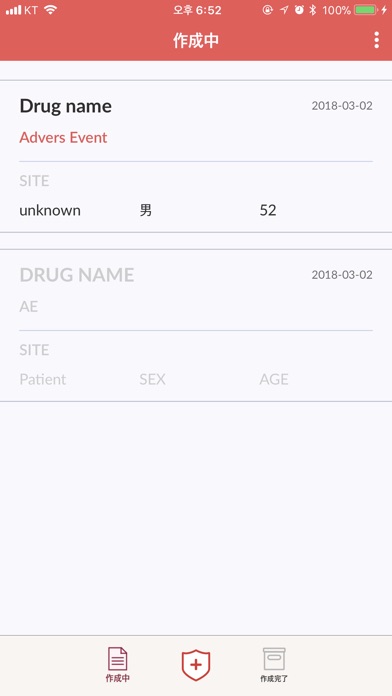
Safety App lets MR (Medical Representative) submit safety reporting with adverse events.
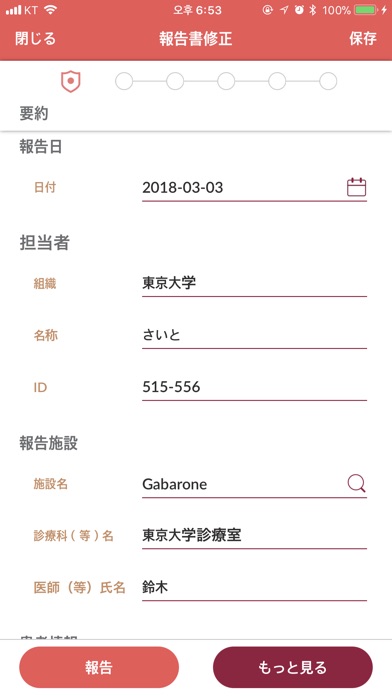
Adverse events which MR found on sites (e.g. hospital) are reported with the following detailed information.
- Site information
- Patient information
- Drug information
After a safety reporting is submitted, both a report (PDF format) and ICSR (Individual Case Safety Report, XML format) are sent to PV (Pharmacovigilance) via Email.